Business
Legal considerations in the age of healthcare and life sciences innovation

Healthcare and life sciences are highly regarded fields that play a big role in modern society. As such, there is ongoing innovation in this sector. Healthcare technology, pharmaceuticals and biotechnology are just some of the areas that contribute to their evolving nature.
Industry professionals have a responsibility to uphold compliance to ensure everything is conducted safely and in line with regulations. Other legal complexities may involve anything from ethical disputes to intellectual property protection.
Consulting with legal experts specialising in healthcare law and life sciences can help to ensure compliance and protect innovation in this highly regulated field. This way, organisations can strike a balance between the two.
Navigating regulatory compliance
When companies come up with new technologies or drugs, they are often faced with challenges around evolving regulations. To introduce new products to the market, they need to create strategies that allow them to meet this criteria.
In the UK, standards are set by authorities such as the MHRA (Medicines and Healthcare products Regulatory Agency) so having a detailed understanding of these ever-changing requirements reduces risks in this area.
Intellectual property protection
Intellectual property (IP) allows organisations to remain competitive. They can allow periods of market exclusivity and ultimately, help companies to maintain market leadership.
Patents allow innovators exclusive rights to their inventions for a specified period. This prevents others from leveraging their patented technology without permission. Meanwhile, trademarks protect brand identity and ensure quality by preventing counterfeit products from entering the market. This is especially important in the pharmaceutical industry to prevent the circulation of fake drugs with serious health consequences.
The safeguarding of trade secrets is another form of IP that shouldn’t be underestimated. This can be put in place to shield confidential information such as manufacturing processes and formulas.
Ethical and legal challenges
As in any case, ethical challenges should be handled with care. Across the industry, there may be controversies around technology such as AI editing or gene editing. Clinical trials also need to be conducted ethically with full disclosure and understanding.
This is where regulatory bodies such as the Health Research Authority (HRA) and the Medicines and Healthcare products Regulatory Agency (MHRA) play an important role. Having a thorough understanding of patient consent and data use is critical.
Data privacy and security
Healthcare is becoming increasingly digitalised. AI-driven diagnostics and digital health records are built into much of the technology used in medical settings. This means there’s an additional layer of compliance that needs to be followed to protect sensitive and confidential information.
Organisations must also comply with GDPR (General Data Protection Regulation) to protect patient data. This can be done through encryption, audits, risk assessments and so on. Fostering a culture where staff are well educated on the matter is another important aspect.

-
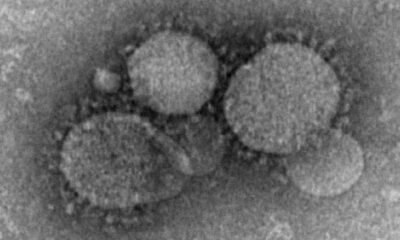
 Health1 week ago
Health1 week agoFrance confirms 2 MERS coronavirus cases in returning travelers
-
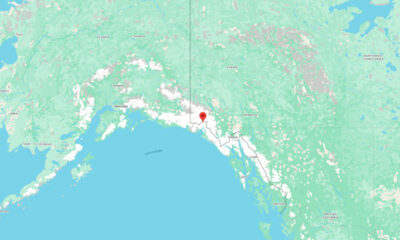
 US News6 days ago
US News6 days agoMagnitude 7.0 earthquake strikes near Alaska–Canada border
-

 Entertainment1 week ago
Entertainment1 week agoJoey Valence & Brae criticize DHS over unauthorized use of their music
-

 Legal3 days ago
Legal3 days agoShooting at Kentucky State University leaves 1 dead and another critically injured
-

 Business2 days ago
Business2 days agoUnpublished TIME cover suggests AI leaders may be named Person of the Year
-

 Legal1 week ago
Legal1 week agoWoman detained after firing gun outside Los Angeles County Museum of Art
-

 Entertainment1 week ago
Entertainment1 week agoSeveral countries withdraw from 2026 Eurovision after Israel is allowed to participate
-
 US News1 week ago
US News1 week agoErroneous earthquake warning triggers alerts across California




