Business
FDA approves ‘first-in-class’ non-opioid painkiller
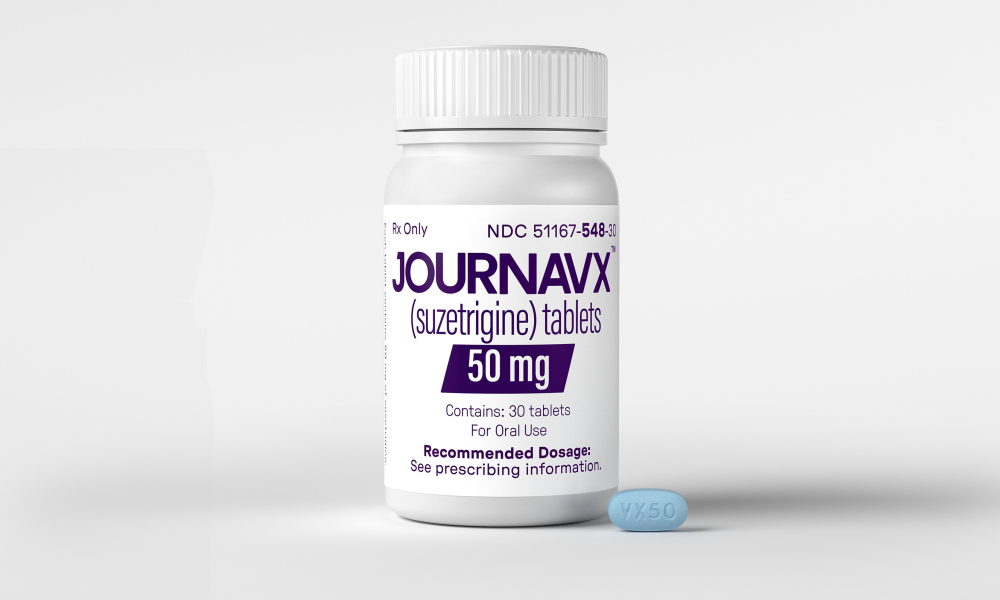
The U.S. Food and Drug Administration (FDA) has approved a non-opioid analgesic for the treatment of acute pain in adults, as part of an effort to encourage the development of non-opioid pain treatments.
The FDA announced on Thursday the approval of Journavx (suzetrigine) 50-milligram oral tablets from pharmaceutical company Vertex, describing it as a “first-in-class non-opioid analgesic” for moderate to severe acute pain.
Journavx reduces pain by targeting a pain-signaling pathway involving sodium channels in the peripheral nervous system before pain signals reach the brain, the FDA said. This contrasts with opioids, which manage pain by binding to brain receptors that receive nerve signals from different parts of the body, but also have addictive effects.
The FDA has issued draft guidance aimed at encouraging the development of non-opioid analgesics for acute pain while working to reduce the clinical practice of prescribing opioids.
“Today’s approval is an important public health milestone in acute pain management,” said FDA Director Dr. Jacqueline Corrigan-Curay. “This action and the agency’s designations to expedite the drug’s development and review underscore FDA’s commitment to approving safe and effective alternatives to opioids for pain management.”
Common side effects of Journavx in study participants included itching, muscle spasms, elevated blood levels of creatine phosphokinase, and rash.
Journavx will cost $15.50 per pill, making it significantly more expensive than comparable opioids, which are often available as generics for less than $1, according to the Associated Press. Although the drug proved more effective than a placebo in clinical studies, it did not surpass the effectiveness of a commonly used opioid-acetaminophen combination pill.
“It’s not a slam dunk on effectiveness,” Michael J. Schuh of Mayo Clinic Florida told AP. “But it is a slam dunk in that it’s a very different pathway and mechanism of action. So, I think that shows a lot of promise.”
“Today’s approval is a historic milestone for the 80 million people in America who are prescribed a medicine for moderate-to-severe acute pain each year,” said Dr. Reshma Kewalramani, CEO and President of Vertex. “With the approval of Journavx, a non-opioid, pain signal inhibitor and the first new class of pain medicine approved in more than 20 years, we have the opportunity to change the paradigm of acute pain management and establish a new standard of care.”

-
 Health1 week ago
Health1 week agoFrance confirms 2 MERS coronavirus cases in returning travelers
-
 US News5 days ago
US News5 days agoMagnitude 7.0 earthquake strikes near Alaska–Canada border
-

 Entertainment1 week ago
Entertainment1 week agoJoey Valence & Brae criticize DHS over unauthorized use of their music
-

 Legal2 days ago
Legal2 days agoShooting at Kentucky State University leaves 1 dead and another critically injured
-

 Legal1 week ago
Legal1 week agoWoman detained after firing gun outside Los Angeles County Museum of Art
-
 Health1 week ago
Health1 week agoEthiopia reports new case in Marburg virus outbreak
-

 Business1 day ago
Business1 day agoUnpublished TIME cover suggests AI leaders may be named Person of the Year
-

 Entertainment1 week ago
Entertainment1 week agoSeveral countries withdraw from 2026 Eurovision after Israel is allowed to participate




