Business
FDA approves ‘first-in-class’ non-opioid painkiller
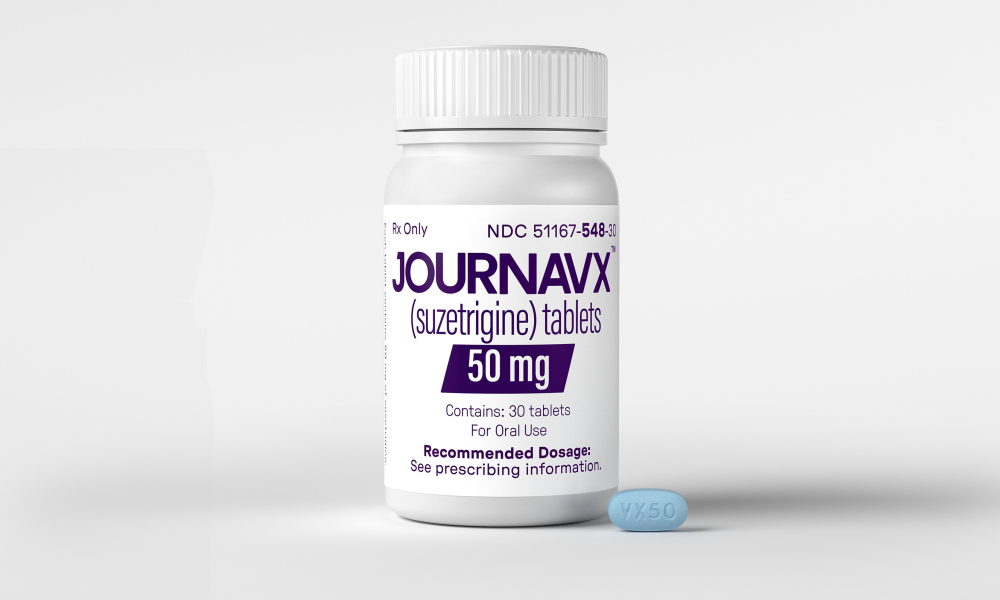
The U.S. Food and Drug Administration (FDA) has approved a non-opioid analgesic for the treatment of acute pain in adults, as part of an effort to encourage the development of non-opioid pain treatments.
The FDA announced on Thursday the approval of Journavx (suzetrigine) 50-milligram oral tablets from pharmaceutical company Vertex, describing it as a “first-in-class non-opioid analgesic” for moderate to severe acute pain.
Journavx reduces pain by targeting a pain-signaling pathway involving sodium channels in the peripheral nervous system before pain signals reach the brain, the FDA said. This contrasts with opioids, which manage pain by binding to brain receptors that receive nerve signals from different parts of the body, but also have addictive effects.
The FDA has issued draft guidance aimed at encouraging the development of non-opioid analgesics for acute pain while working to reduce the clinical practice of prescribing opioids.
“Today’s approval is an important public health milestone in acute pain management,” said FDA Director Dr. Jacqueline Corrigan-Curay. “This action and the agency’s designations to expedite the drug’s development and review underscore FDA’s commitment to approving safe and effective alternatives to opioids for pain management.”
Common side effects of Journavx in study participants included itching, muscle spasms, elevated blood levels of creatine phosphokinase, and rash.
Journavx will cost $15.50 per pill, making it significantly more expensive than comparable opioids, which are often available as generics for less than $1, according to the Associated Press. Although the drug proved more effective than a placebo in clinical studies, it did not surpass the effectiveness of a commonly used opioid-acetaminophen combination pill.
“It’s not a slam dunk on effectiveness,” Michael J. Schuh of Mayo Clinic Florida told AP. “But it is a slam dunk in that it’s a very different pathway and mechanism of action. So, I think that shows a lot of promise.”
“Today’s approval is a historic milestone for the 80 million people in America who are prescribed a medicine for moderate-to-severe acute pain each year,” said Dr. Reshma Kewalramani, CEO and President of Vertex. “With the approval of Journavx, a non-opioid, pain signal inhibitor and the first new class of pain medicine approved in more than 20 years, we have the opportunity to change the paradigm of acute pain management and establish a new standard of care.”

-

 Legal7 days ago
Legal7 days agoFirefighters ambushed while responding to Idaho wildfire, at least 2 killed
-

 Legal1 week ago
Legal1 week agoWashington Post journalist Thomas LeGro arrested for child porn possession
-

 World1 week ago
World1 week agoTropical Storm Barry forms in the Gulf, expected to make landfall in eastern Mexico
-

 Legal5 days ago
Legal5 days agoOvidio Guzmán, son of ‘El Chapo,’ to plead guilty in Chicago drug trafficking case
-

 US News5 days ago
US News5 days agoMassive explosions reported at fireworks site in Yolo County, California
-

 Legal4 days ago
Legal4 days agoAt least 4 injured in shooting at mall in Savannah, Georgia
-
 Health5 days ago
Health5 days agoCambodia reports 3 new human cases of H5N1 bird flu
-

 US News1 week ago
US News1 week agoU.S. ends Temporary Protected Status for Haiti, citing improved conditions



