World
WHO approves first mpox diagnostic test for emergency use
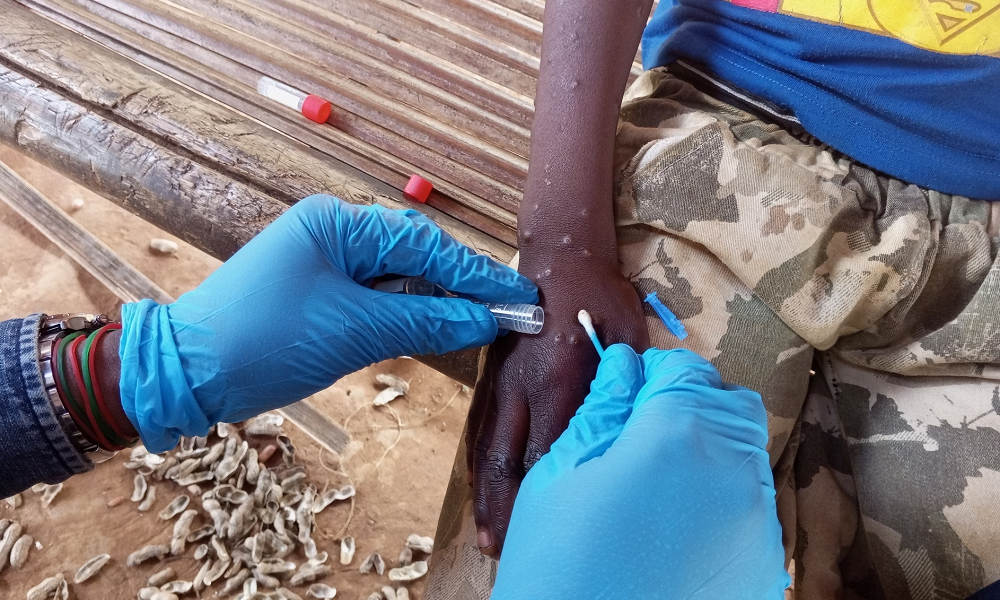
The World Health Organization has approved an mpox diagnostic test developed by Abbott Laboratories for emergency use, which should expand access to tests in efforts to contain a growing outbreak in Africa which has infected tens of thousands of people.
The Alinity m MPXV assay, which is meant for use by trained clinical laboratory personnel, is a real-time PCR test that enables the detection of monkeypox virus DNA from human skin lesion swabs. This allows suspected cases to be confirmed in an efficient manner.
“This first mpox diagnostic test listed under the Emergency Use Listing procedure represents a significant milestone in expanding testing availability in affected countries,” Dr. Yukiko Nakatani, a WHO official, said in a statement. “Increasing access to quality-assured medical products is central to our efforts in assisting countries to contain the spread of the virus and protect their people, especially in underserved regions.”
While this is the first diagnostic test to receive emergency use authorization for mpox, WHO is also considering three additional submissions to further expand access to faster and efficient testing.
In August, the World Health Organization declared mpox a Public Health Emergency of International Concern. The emergency use listing for Alinity m MPXV assay will remain valid as long as the health emergency remains in effect.
More than 30,000 confirmed and suspected cases have been reported across Africa so far this year, an increase of nearly 200% compared to 2023. At least 738 people have died. In one of the hardest-hit countries, the DR Congo, only 37% of suspected cases were able to get tested.
Of particular concern is clade 1b, which is causing more severe disease compared to the variant which spread around the world in 2022. It’s also believed to be more infectious. Clade 2b mainly spread through sexual contact while the new strain is more commonly spread through other contact routes, including close contacts within households.
The test developed by Abbott Laboratories is able to detect both variants.
Several vaccines have already been approved for mpox but only few people have had them so far, especially in Africa. WHO has triggered the process for Emergency Use Listing for two vaccines to make them more accessible, particularly in lower-income countries.

-

 World1 week ago
World1 week agoEthiopian volcano erupts for first time in thousands of years
-

 Legal5 days ago
Legal5 days agoUtah Amber Alert: Jessika Francisco abducted by sex offender in Ogden
-

 US News4 days ago
US News4 days agoExplosion destroys home in Oakland, Maine; at least 1 injured
-
 Health4 days ago
Health4 days agoMexico’s September human bird flu case confirmed as H5N2
-

 World5 days ago
World5 days agoWoman killed, man seriously injured in shark attack on Australia’s NSW coast
-

 Legal19 hours ago
Legal19 hours ago15 people shot, 4 killed, at birthday party in Stockton, California
-

 Legal1 week ago
Legal1 week agoKnife-wielding man shot dead by police at St. Louis airport
-
 US News4 days ago
US News4 days agoEarthquakes rattle area between Salinas and Hollister, California




